The End Of Antibiotics; The Rise Of Bacteria
We have a serious problem. If you think Covid is bad… buckle up!
The empirical application of moldy bread on wounds started long before the concept of bacterial infection was even understood. There are many historical references to ancient civilizations’ healers using antibiotics.
They did not know about the existence of bacteria, only that after throwing whatever they had handy on wounds, some things anecdotally worked better than others… I am guessing this kitchen-sink approach to medicine killed more people than they saved, but sometimes it worked, and when it did, healers would try the same technique more often.
The first direct documentation on molds to treat infections was by John Parkinson in the 16th century. However, it took four centuries until Alexander Fleming discovered modern-day penicillin (1928).
Easy access to antibiotics changed the world. All of a sudden, a lot fewer people died of common infections. Can you imagine a world where every open wound was death threatening? That was pretty much what we had before penicillin. Having a healer applying random stuff on your body that could either save you or be the source of the infection was the only option besides waiting and doing nothing.
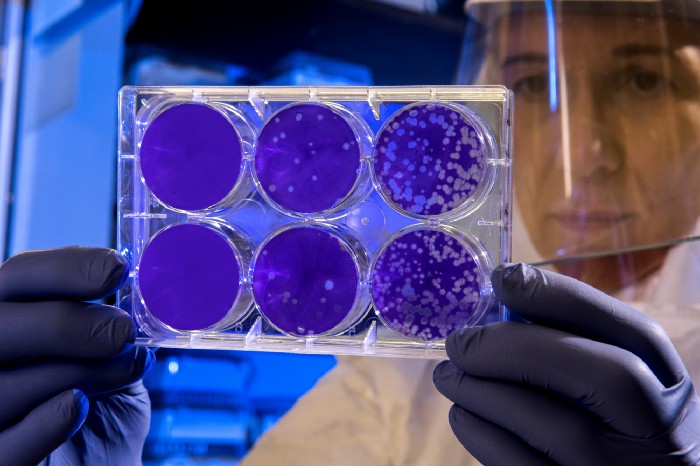
Antibiotics were massively overused, and some bacteria have evolved resistance to them. Some bacteria, mainly in hospital settings, have resistance to all known antibiotics. That means modern medicine gets medieval once again. We resort to amputation of localized infected limbs or simply wait for a miracle recovery with nothing much we can do besides observing the patients.
Antimicrobial resistance is a widespread threat that is no longer a prediction for the future; it is happening right now.
Unless we put some serious effort into developing new antibiotics, we are heading into a very different world than the one we currently live in. But, unfortunately, there are no guarantees of success even if we apply resources to the problem.
Want to look at the faces of frustrated doctors and dying patients? There is this excellent documentary by PBS — “When Antibiotics Don’t Work”.
Alternatives to currently used Antibiotics?
Most antibiotics we currently use are either natural products or derivatives. Scientists are continuously screening nature in a quest for the next batch of antibiotics or products that suppress/reduce antibiotic resistance and antibiotic tolerance in bacteria. Stopping bacterial virulence factors (that allow bacteria to circumvent our body’s defenses) is another possible avenue.
We are losing that race against acquired bacteria-resistant to our currently available solutions. But, unfortunately, we keep reducing the landscape of solutions yet undiscovered by science with our actions that lead to degradation and extinction of natural ecosystems.
Monoclonal antibody treatments became well known to many during the Covid pandemic. The generic concept is that we create antibody’s in a different host (like mice) and export them in a cocktail prepared for human consumption. Unfortunately, creating Monoclonal antibody cocktails is not cheap and hard to produce in large quantities since you have to farm the original hosts. Still, it’s a possible avenue that science is exploring.
Another approach to treating antibiotic-resistant strains of bacteria is Phage therapy. We are using viruses to infect particular bacteria and kill them. Possible disadvantages of this approach are that we may be opening Pandora’s box; Bacteriophages may have undesired virulence factors or toxic genes in their genomes. In addition, there is a risk of adverse immune responses from our human bodies to treatment. Nevertheless, the use of bacteriophages against pathogens that no longer respond to conventional antibiotics is seen as an attractive last resort despite all the regulatory hurdles and risks since we are running out of options.
RNA-based treatment (gene silencing therapy) synthesizes complementary single-stranded antisense RNA (that will insert itself) to the mRNA encoding essential bacterial genes proteins. The aim is to suppress the bacteria genes responsible for antibiotic resistance. It is demoting the super-bugs to lesser versions of themselves.
There is hope in science. But the odds are stacked against us. The economic incentives will undoubtedly play a big part in the outcome, research and development are not cheap, and investments are risky.




